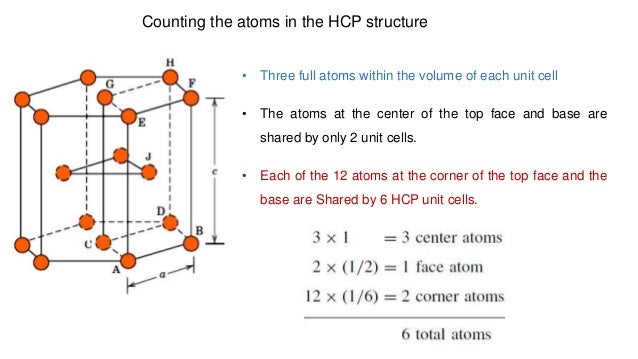
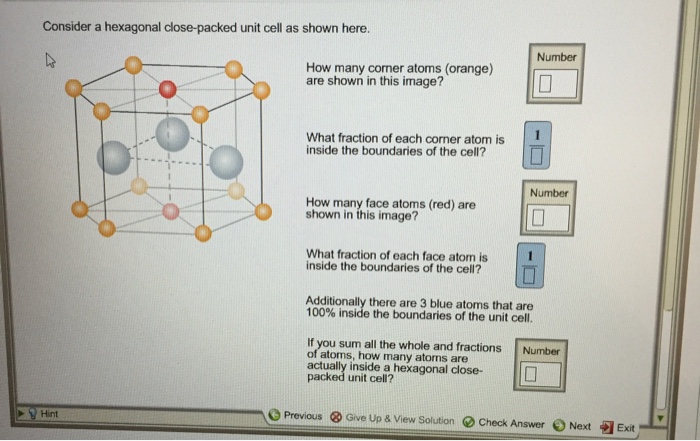
A more challenging task is to determine the number of atoms that lie in the unit cell. Autodesk t splines plug in for rhino download. As described above, an atom is centered on each corner, there is an atom centered inside the unit cell (but this atom extends outside the unit cell), and there are two atoms centered outside the unit cell that extend into the unit cell. Number of atoms in hexagonal close-packed (HCP) unit cell. Whenever the atomic packing factor for the hexagonal close-packed (HCP) crystal structure is discussed, such as in this wikipedia article, it is stated that the (effective) number N of atoms in a unit cell (chosen as a hexagonal prism) is 6 - corner atoms contribute 1 / 6 each, face-centred atoms contribute 1 / 2 each, and middle-layer atoms contribute 1.
What is the total volume of an HCP unit cell? Hexagonal close packing is contain 17 atoms 6 is below side in hexagonal and 6 is up side and also both face center contain 1–1 atom. Thereafter 3 atoms are in hexagonal close packing so total 17 atoms. The number of atoms in the hcp unit cell is 6. Project online desktop client mac. 3 atoms are placed in the middle of the unit cell. The contribution of each such atom to the unit cell is 1. 2 atoms are present at two face centers. The contribution of each such atom to the unit cell is one half. 12 atoms are present at 12 corners.
Kumar Sarang answered this
How Many Atoms In Hcp Unit Cell
Number Of Atoms In Hcp Unit Cell Wall
The top and bottom layers consists of six atoms each arranged in a hexagonal shape.Each of these atoms have a contribution of 1/6.
The seventh atom is placed at the centre of this hexagonal arrangement of atoms at the top and as well as at the bottom.These two atoms have a contribution of 1/2 each.
The centre layer consist of three atoms arranged in a triangular form .Each of them have a contribution of 1.
Thus total no. of atoms in hcp = 1/6 x 12 +1/2 x 2 +1 x 3 =6 atoms
Thus
Z= 6 for hcp
The packing fraction for hcp is same as that of fcc i.e 0.74.
